Neuroanalysis of Therapeutic Alliance in the Symptomatically Anxious: The Physiological Connection Revealed between Therapist and Client
Abstract
This study was an attempt to establish neurophysiological correlates, particularly brain activity, during high therapeutic alliance (TA) between client and therapist. The aim was to assess electroencephalography (EEG) activity in clients with symptomatic anxiety during high TA using skin conductance resonance measurements from both client and therapist. Thirty clients, aged 43.8 ± 11.5 years (males: n = 15 females: n = 15), underwent six, weekly, 1-hour sessions (180 hours of repeated measures). The EEG activity was measured from the prefrontal, temporal, parietal and occipital sites during the sessions. State and trait anxiety, Working Alliance Inventory (WAI) and heart rate measures were obtained before and after each session. Prefrontal, parietal and occipital sites were associated with TA. Anxiety and heart rate were found to decrease after therapy, and for both the client and the therapist, the WAI score increased significantly in later sessions. The results are discussed from the perspective of further understanding the neurophysiological associations to TA.
Introduction
The core concept of therapeutic alliance (TA) is a key component of psychotherapy. It was defined by Freud (1912), as the sine qua non of therapy, now referred to as empathy, which is both a state of being and a therapeutic skill (McLeod, 1998). Freud understood TA to be closely linked with transference (Sandler, Dare & Holder, 1973). The transferential aspect was eventually separated from TA, with the alliance becoming known as the “working alliance” (Greenson, 1967). Inadequate and empirically inept methods kept the therapeutic alliance from being extensively investigated as a clinical construct until the mid 1970s (Horvath & Greenberg, 1994). The term, therapeutic alliance, contextualizes the relational dimension between client and therapist (Horvath, Gaston & Luborsky, 1993) and encompasses the therapeutic framework and mutual responsibilities between therapist and client which are crucial to a positive clinical outcome (Meissner, 1992). It is described as the intersubjective relational field in which evolutionary growth is seen as an outcome (Stolorow, Brandchaft & Atwood, 1987). Lewis, Amini and Lannon (2000) call therapeutic alliance a deep empathic state emanating from the limbic part of the brain, which allows us to be aware of the emotional state of others. The limbic system of the brain is involved with recording and accessing emotional memory, learning, and the mediation of our primitive flight/fight avoidant responses (Patterson & Schmidt, 2003). The limbic brain stores emotional experiences in the unconscious creating implicit or unconscious memory which is not under cortical control (Stolorow, 1994; Lewis, Amini & Lannon, 2000). Through TA therapists access this implicit memory which helps clients to access and regulate their emotions and ultimately revise their lives (Schore, 1994; Caspi, McLeay & Poulton, 2002). It is only in this type of therapeutic relationship that the client can redefine the concept of self by opening to the possibility of evolutionary change (Yalom, 1980) or healing the self-system (Kohut, 1977). To create this moment of meeting’ (Stern, 2004), or intersubjective system of reciprocal mutual authority (Stolorow, 1994), therapists need to access their intuitive subjective responses as well as their objective theoretical knowledge (Schore, 2003b).
A successful therapeutic relationship engenders a positive mood regulating context that encourages development in the instinctive right brain of both the therapist and client, in which out-of-conscious meaning making evolves (Schore, 2003a, p. 38). The relationship between client and therapist during the therapeutic alliance is both empathic and dynamic, where interpersonal problems are confronted (Howard et al., 2006). This essential corrective interpersonal experience with the therapist can be challenging (Paivio & Shimp, 1998), involving the expression of both positive and negative emotions (Gottman & Krokoff, 1989). Our study showed the higher the levels of anxiety in clients the greater the development of therapeutic alliance.
In this exploratory study, therapeutic alliance is classified according to Horvath and Greenberg’s (1994) definition of goal, task, and bonding principles (Horvath & Greenberg, 1994, p. 15). Research shows that a good quality therapeutic alliance is the most common element for positive client change in all models of psychotherapy (Smith, 1990; Horvath & Symonds, 1991; Lambert, 1992; Lambert & Hill, 1994; Horvath, 2000; Kaufman, 2000; Hendricks, 2002; Summers & Barber, 2003). Therapeutic alliance is now known to be a key factor of therapeutic change by enabling clients to develop new neural networks through integrating new thoughts, feelings, and behaviours (Cozolino, 2002). Recent literature shows neural firing follows attention (Siegel, 2006). Grounded emotional functioning modeled by the psychotherapist is experienced and integrated on a neural level by the client through the therapeutic relationship (Lewis, Amini & Lannon, 2000). In this study we focused on establishing the neurophysiological correlates of therapeutic alliance in a symptomatically anxious client group.
Anxiety and fear are the intentional features of our continual evaluations of dangers that effect significantly our thoughts or cognitive functioning, feelings, and emotions (Charney, 2004), and hence, activate our sympathetic nervous system. The amygdale, situated in the temporal lobes and the bed nucleus of the stria terminalis, are the areas in the brain that appear to regulate the control of fear and anxiety (Davis, 1997). During high therapeutic alliance there seems to be less activity in the amygdale. This reduced activity may be indicative of clients being able to reexperience fears and anxieties developed earlier in life in a corrective, interpersonal relationship The above literature shows that very little data exists in understanding the neurophysiological changes that may be associated with high therapeutic alliance and the subsequent impact on anxiety levels in symptomatically anxious clients.
Anxiety disorders have a lifetime prevalence of 16.6% worldwide (Somers, Goldner, & Waraich, 2006). Mental illness was the leading disease in Australia in 2005 (Australian Institute of Health & Welfare, 2008). It accounted for 13% of the total health/disability burden in a study undertaken by the Australian Institute of Health and Welfare (AIHW, 2001). Forty-six percent of all the subjects in the report stated they experienced anxiety, defined as a persistent state of apprehension accompanied by signs of physiological arousal, which can be both short and long-term (Andreassi, 2000b, p. 373). Twelve percent of adolescents aged more than14years reported an anxiety attack in the last year, of the 12% of adolescents, 14% were treated for a mood disorder including depression and bi-polar disease, 28% had experienced diagnosed stress and 9% had panic attacks (Benjamin, 2007)
Our preliminary study, in which 15 symptomatically anxious clients underwent six therapy sessions of one hour duration (total 90 hours of therapy), we established that the frontal site of the brain remained active during high TA, while the occipital site “went to sleep” (Stratford, Lal, & Meara, 2009). During high TA the parietal cortex–the “seat of imagination”—was extremely active, especially when the client appeared to be intuiting cognitive and emotional insight. The temporal site reflected the client accessing emotional memory with high alpha and beta activity. Heart rate (HR) and anxiety levels were found to decrease over the course of the six sessions. This study established some initial neurophysiological changes associated with positive therapeutic outcome.
The aim of this research was to further the study by Stratford, Lal & Meara, (2009) and to establish the neurophysiological changes in symptomatically anxious clients undergoing therapy, during moments of high TA. Therapeutic alliance was assessed using skin conductance resonance (SCR) measurements from both client and therapist (Marci et al., 2007).
Methods
Clients
Clients (N=30; 15 men and 15 women) participated in the study. The exploratory clinical study had the Institute’s ethics approval. All volunteers were recruited from the community via flyers, posters and community health centres. Demographic data from the random sample included self-report of age, gender, marital status, medical diagnosis, current medication, and ethnic background (Craig, Hancock & Craig, 1996), which reflected the multicultural makeup of New South Wales, Australia.
All subjects reported experiencing medium-to-high levels of anxiety, which was evaluated prior to the commencement of the clinical psychotherapy sessions using the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1983) to establish level of state and trait anxiety. The self-report anxiety questionnaire has high internal consistency and validity (Cronbach’s alpha, 0.93).
Clients completed an informed consent at the beginning of the study and questionnaires at the beginning and end of each session. The questionnaires took 5 minutes to complete before each session and 10 minutes at the end.
Before the start of therapy, participants were screened to ensure no medical contraindications, such as severe concomitant disease, alcoholism, drug abuse and psychological or intellectual problems, existed that could limit compliance. This was determined during the initial interview using the Lifestyle Questionnaire (Craig, Hancock & Craig, 1996), which has high internal consistency and validity (Cronbach’s alpha, 0.89). There were no dropouts, with all clients completing their allocated six sessions.
Five registered clinical psychotherapists and one clinical psychologist (3 women and 3 men) provided the process-orientated psychotherapy. Therapists reflected approaches coming from diverse modalities, including gestalt, psychodynamic, psychosynthesis, person-centred and somatic psychotherapy, but used a standard relational client-centred method of working.
Study Protocol
The therapy was carried out in a laboratory setting with clients completing 6 psychotherapy sessions, once per week for 6 weeks. Each session was 45 to 60 minutes in duration. This study reflects 180 hours of total therapy sessions (preliminary findings were with 15 clients x 6 sessions which gave 90 hours; this paper covers the entire 30 clients x 6 sessions which gives 180 hours).
Physiological and Subjective Measures
Physiological Measures
Simultaneous physiological measures were collected during each session using a physiological monitor, the Flex Comp (Infinity, Thought Technology, Ltd., USA). Electroencephalogy (EEG), electrocardiogram (ECG) was recorded from four sites on the client and one site on the therapist. A third-order Butterworth band pass filter was applied (1.5Hz to 40Hz) to remove any noise artefacts from the EEG data.
Skin conductance resonance (SCR) response from the therapist and client was recorded continuously over the session to establish the therapeutic index (TI), according to guidelines given in Marci et al (2007). The TI was computed by assessing 5-second slopes at 1-inch intervals rather than from peak to peak, giving a continuous, not an intermittent, reading of the autonomic nervous system (Oppenheim & Schafer, 1999). Four-channel EEG was recorded according to the International 10-20 System (Fisch, 1991). The EEG electrodes were placed at the prefrontal (F1, F2), temporal (T7, T8), parietal (P3, P4) and occipital (O1, O2) sites. Each EEG electrode mount (EEG-Z, Model, Thought Technology, USA), was filled with electrode gel (Signa Gel, Parker Laboratories, USA). The reference ear-clip electrodes for the EEG signals were positioned on both ear lobes. Three-lead ECG was recorded using electrodes (EKG-Flex/Pro, Model SA9306M, Thought Technology, USA) placed on the chest, one reference and two active. Skin conductance resonance (SC-Flex/Pro, Model SA9309M, Thought Technology, USA) was measured from both the therapist and the client with electrodes taped around their index and middle fingers. The therapeutic index (TI) was calculated according to Marci et al. (2007; refer to analysis section below), which was determined from the SCR measurements of both the client and the therapist. Sterile techniques were used for all procedures, which were painless causing minimum irritation. Heart rate (HR) was recorded before and after each session using a digital monitor (Omron, Model M5 (HEM-742C-C1), Omron Corporation, USA).
Subjective measures
Clients completed the Speilberger State Trait Anxiety Inventory criteria ([STAI]; Speilberger et al., 1983), which determines trait and state anxiety norms for the general population. State anxiety establishes current or “here-and-now” anxiety norms while trait anxiety evaluates long-standing anxiety. Anxiety states are defined by subjective feelings of tension, apprehension, nervousness, which stimulates the autonomic nervous system. Trait-anxiety refers to consistent individual differences in anxiety proneness, in which moderately stressful events are interpreted as dangerous or threatening and the response to these situations increases the strength of state anxiety reactions (Andreassi, 2007). A client’s trait anxiety was determined pre-study, and state anxiety was established both pre- and post-session.
The long version of the working alliance inventory (WAI) (Horvath & Greenberg, 1986) was completed at the end of each session by both client and therapist, with a completion rate of 100%. The WAI, by Horvath & Greenberg (1986), is a 36-item, self-reported questionnaire rated on a 7-point-Likert scale, comprising of three subscales of task, bond, and goal, with a high internal consistency (range = .87-.93). Cronbach alpha for bond is .92; for task, .92; and for goal, .89. The WAI is one of the first validated instruments to subjectively measure therapeutic alliance (Hanson, Curry & Bandalos, 2002), and it evaluates both client and therapists perceptions on agreement of goals, assignments of tasks, and the development of a bond, which are acknowledged as the essential ingredients of the therapeutic alliance (Bale et al., 2006).
Analysis
The EEG data was sampled at 1000 Hz and then sectioned into epochs of 1-second, which was then subjected to a fast Fourier transform algorithm to obtain a single value for each of the four EEG frequency bands. The EEG was classified in terms of four frequency bands including delta (0-4 Hz), theta (4-8 Hz), alpha (8-13 Hz) and beta (13-20 Hz) (Fisch, 1991). For each band the average EEG power (μV) was computed for four sites: prefrontal (F1, F2), temporal (T7, T8), parietal (P3, P4), and occipital (O1, O2). Heart rate was derived in number of beats/minute.
Therapeutic index (TI), a measure of physiological concordance between client and therapist (Marci et al., 2007), was computed from the natural logarithmic value of the ratio of the sum of the positive SCR values, divided by the absolute sum of the negative values. The highest TI found across 3-minute segments of the session (which reflected the greatest therapeutic alliance between client and therapist) was used to observe the simultaneous effects of high TA on other neurophysiological data (EEG and HR activity). High TI indicated positive concordance (TA) between client and therapist. All neurophysiological data was analysed using software developed in house (creators: Andrew Varis and Budi Jap).
The study was an exploratory design and data analysis was performed using Statistica (for Windows, V 8, StatSoft, USA). Pre- and post-session differences were computed using independent t-tests for state anxiety, HR, and BP data. Differences in post-study WAI scores between client and therapist were also compared using a t-test. State anxiety, EEG, HR, and WAI were also compared across the 6 therapy sessions using analysis of variance (ANOVA). The analysis design was a within-subject ANOVA factor, high TI × the six therapeutic sessions, which compared the absolute TI scores as well as the EEG and HR scores. A less conservative Fischer LSD test was used to determine specifically where differences existed among the 6 sessions. The Fisher LSD is a post-hoc analysis test for multiple comparisons and is used to identify where significant differences exist between the means of the different groups originally subjected to an Analysis of Variance Test (Statistica for Windoes, V8, Statsoft, USA). Significant results at a p value of <0.05 are reported as well as trends in data for p<0.1.
Results
Thirty clients completed the study and participated in six therapy sessions, reflecting a total of 180 hours of therapy. The fifteen men and fifteen women were aged 43.8 ± 11.4 years, with a BMI of 23.6 ± 2.6. The average sample trait anxiety (long-term anxiety) was 48 ± 10.3. The norm reported for men in this age group is 35.1 ± 8.9, and for women 35 ± 9.3 (Spielberger et al., 1983). This demonstrated that results for the participants in this study were indicative of a group that was symptomatically anxious.
Figure 1 shows the average state anxiety changing across the 6 sessions (labeled S1 to S6) for both pre- and post-session. Presession state anxiety was significantly lower in S6 (33.6 ±11.3), compared to S 1 (41.6 ± 11.1; p<0.004). There was also a trend of presession state anxiety to be lower in S4 (p=0.05) and 5 (p=0.07) compared to S1. Postsession state anxiety decreased from S2 (35 ±10.8) to S6 (29.4 ± 7.6; p=0.03). Results revealed a general lowering of anxiety across the 6 sessions. After comparing state anxiety scores before and after each session using a t-test, it was established that anxiety was significantly reduced at the end of all sessions except session 2.
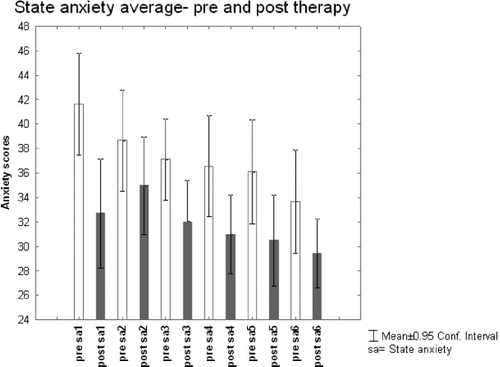
Figure 1. PRE AND POST THERAPY AVERAGE STATE ANXIETY
| S1 | T=3.5 | df=29 | p=0.001; |
| S3 | T=2.9 | df=29 | p=0.007 |
| S4 | T=4.0 | df=29 | p=0.0004 |
| S5 | T=3.7 | df= 29 | p=0.0008 |
| S6 | T=2.6 | df= 29 | p=0.01 |
Table 1. S2, Anxiety not significantly different before and after the therapeutic session.
Figure 2 shows moments of high concordance as recorded from the SCR (representing high TA) of both therapist and client. Figure 2 also shows the moments of low concordance for comparison. During moments of high TA, the frontal site showed lower beta activity during S6 compared to S3 (p=0.045). Parietal theta activity reduced from S1 to the later sessions (S3 and S5, p = 0.02; S6, p=0.01). In the parietal region, alpha activity also reduced in later sessions (S2, p=0.49; S3, S5, S6, p=0.02). Occipital beta showed greater activity during session 4 as opposed to session 3 (p=0.045).
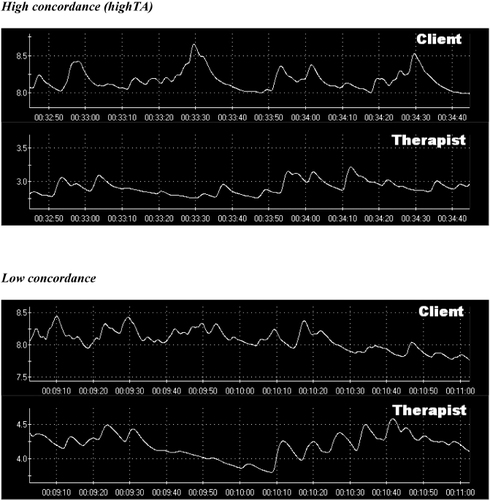
Figure 2. HIGH AND LOW CONCORDANCE OF THERAPEUTIC ALLIANCE ACCORDING TO SKIN RESONANCE CONDUCTANCE MEASURE IN CLIENT AND THERAPIST
Trends in the brain data were also identified and revealed that delta activity reduced in the frontal region being lower in S6 as compared to S1 (p=0.09). There was an increase in frontal alpha (p = 0.07) in S3 compared to S2. Alpha activity in the frontal region then reduced from S3 to S4 (p=0.07). During S3 there was higher frontal beta activity as opposed to S4 (p=0.06). Compared to S1, temporal theta was lower in S3 (p=0.09), S4 (p=0.056) and S5 (p=0.08). The parietal region had higher beta activity during S4 as compared to S6 (p=0.058). Occipital theta was higher in compared to S2 (p=0.08), S3 (p = 0.07), and S6 (p=0.09). In S4 occipital beta activity was higher compared to S2 (p=0.07) and S6 (p=0.06).
Heart rate (HR) reduced significantly after each therapy session compared to before the session (p<0.001). Figure 3 shows a trend for HR decreasing from S1 to S6 both pre- and post-therapy sessions (pre-S1, 79 beat per minute (bpm) to pre-S6, 77 bpm; and post-S1, 73 bpm to post-S6, 70 bpm).
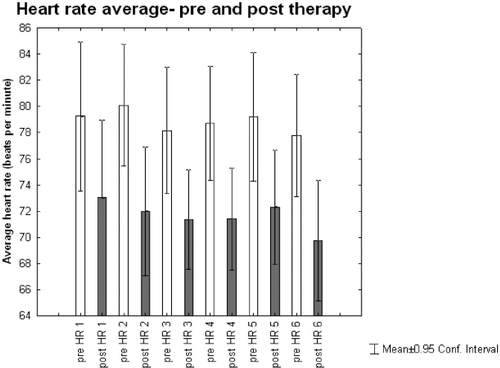
Figure 3. PRE AND POST THERAPY AVERAGE HEART RATE (HR)
The ANOVA revealed an overall difference in the WAI score for clients across the six therapy sessions (F= 28829, df=5, 174, p<0.0001, refer to Figure 4). The WAI scores in sessions 3, 4, 5 and 6 (p=0.047, p = 0.001, p<0.0001, p<.0001, respectively) were significantly higher than in S1 (refer to figure 4 for the average changes in the WAI scores). The average WAI scores per session mean±SD - standard deviation S1: 72.9±7.6; S2: 73.9±7.7; S3: 76.0±6.1; S4: 78.0±5.5; S5: 79.5±4.2; S6: 80.4 ±4.4. The WAI scores in S4, S5, S6 (p=0.009, p<0.0001, p<0.0001, respectively) were significantly lower than in S2 (refer to values above and Figure 4). They were higher in S5 and S6 compared to S3 (S5, p=0.03; S6, p=0.006).
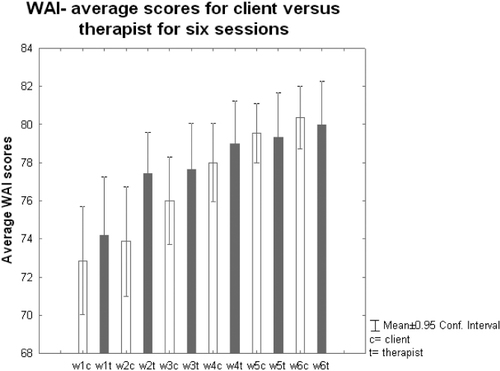
Figure 4. AVERAGE WORKING ALLIANCE INVENTORY SCORES AFTER EACH SESSION FOR CLIENT AND THERAPIST
Similarly there was an overall difference in the WAI score for the therapist across the 6 sessions (F=25437, df=5, 174, p=0.01). For the therapist, the WAI score also increased significantly in later sessions (S3 to S6) compared to S1 (S3, p = 0.04; S4, p=0.005; S5, p = 0.002; S6, p=0.0007).
84 J32 f 80
Discussion
This exploratory study examined the links between high levels of therapeutic alliance and the neurophysiology of symptomatically anxious clients during therapy. The central feature of this study showed that high TA impacted both the client’s brain and body in a unique and profound way. However, it is accepted that the brain is a complex organ and our conclusions are, therefore, necessarily speculative.
EEG
Results of this exploratory study demonstrated that distinct parts of the brain are activated in clients during high TA and that a subsequent related neurophysiological response is seen in this state of ‘flow’ (Cooper, 2005). In a previous study the authors illustrated that during high TA the prefrontal site showed an increase in relaxed wakefulness as therapy progressed (Stratford, Lal & Meara, 2009). This would indicate that the prefrontal cortex is relaxed and not in a state of alarm or activation during moments of high TA, supporting the view that the prefrontal lobes have
the ability to suppress basic drives and feelings while allowing us to strategise or think our way through an issue (Solms, Turnbull & Sacks, 2002).
The parietal cortex was the most significant part of the brain to respond to moments of deep empathic connection or high TA during therapy. It became more active, especially in S4, with an increase in fast-alert beta waves while theta waves (drowsiness) and alpha relaxed wakefulness (Andreassi, 2000a), decreased. Beta waves are known to increase when we access deep levels of unconscious material (Janov, 1996).
Our results showed that once TA is established the parietal lobe becomes active and therefore critical to the emotional and cognitive aspects of insight gathering and the creation of new meaning making in resolving inter and intra-personal issues. The parietal lobe is where we establish focused attention and coordination of all sensory information giving us a sense of space and an ability to read our environment (Veitch, 2008). Research is beginning to show that during periods of focused attention, like high TA, there could be a move from the frontal cortex to the parietal lobes as we process unconscious emotional and cognitive material into consciousness (Cozolino, 2002). This appears to be reflected in our study, with prefrontal slowing and parietal becoming more active. The parietal cortex also directs attention towards visual and emotive stimuli (Fink et al., 1996). Our research shows that this may be happening with a decrease in temporal and occipital alert activity. The parietal cortex is also known as “the seat of imagination” (Decety & Chaminade, 2003). As our research shows significant alert beta activity in this part of the cortex it may indicate that once high TA is established, clients sense enough emotional safety through the suppression of the prefrontal and a relaxed temporal region to focus on their internal capacity to imagine a new future or a way through their distress. Before imagination takes place we need to have established an emotional connection and gained understanding (Grager et al., 2006). We know the brain cannot distinguish between what it imagines and what it experiences. It is interesting to note that when a life-size replica of Einstein’s brain was created, the parietal cortex was not divided by a deep groove (like most) and had increased neuronal connectivity (Lythgoe, 2008). During moments of high TA, our clients experienced increased activity in the parietal cortex, and we hypothesise that this is the part of the brain that may be crucial in problem solving and insight gathering. Our results establish that the parietal cortex shows highly significant activity during S4, after TA has been established by S3. This replicates a growing body of research indicating that TA must be formed by S3 for successful therapeutic outcome (Miller, Duncan & Hubble, 1997; Heaton, 1998).
Demonstrated specific brain activity during therapeutic alliance supports the notion of cortex involvement during the process of therapeutic change. This involves clients being able to access unconscious cognitions and emotions so that maladaptive meaning making can be changed through a corrective interpersonal relationship based on a positive therapeutic alliance (Paivio & Shimp, 1998). The occipital cortex showed higher alert beta activity during S4 with a trend towards more alert activity over the subsequent two sessions. Beta activity increases in the occipital cortex when unconscious material is bought to consciousness (Hebert & Lehmann, 1977). The occipital site also showed an increase in the hypnagogic state of theta, which suggests that the clients attention was focused inward to gain a subjective sense of their mind (Solms, Turnbull & Sacks, 2002, p. 273). Increased theta activity in the occipital cortex has been found to be associated with problem solving (Schacter, 1977). This would suggest that while processing into consciousness emotional and cognitive material, a client may appear to be looking at the therapist but is not seeing him as attention could be focused inward.
The temporal site reflected a trend towards lower theta cortical activity across the sessions indicating the temporal region was attentive but relaxed. A recent fMRI study of anxious clients showed the limbic system, which is located in the temporal cortex and activated by perceived threatening stimuli, was reduced during psychodynamic psychotherapy (Beutal, 2006). When we recognise emotional words, there is also a fall in theta waves in the temporal cortex (Dixon & Lear, 1964). High TA may account for the lack of significant alert activity in the temporal cortex, adding to the body of research indicating this calm state is required for accessing insight (Valentino, Arruda & Gold, 1993). Our results suggest that during moments of high TA, the temporal cortex remains in a “normalised” or optimal state (Farrow, Hunter & Wilkinson, 2005) so other parts of the brain can work on processing trauma and gaining insight. Interestingly the temporal region processes sound and language (Andreassi, 2000a). During moments of high TA, clients appeared to be inwardly focused, processing their thoughts, which is demonstrated by a decrease in talking and an increase in silence.
This study hypothesised that during moments of high TA there would be an impact on the brain and the physiology of clients reinforcing the understanding that the Cartesian split does not exist and that our minds and bodies are one dynamic system (Mackewn, 1997). The impact on client physiology in this study is reflected in outcomes of reduced anxiety.
Anxiety
All 30 clients who participated in this study scored above average for long-term anxiety, which reflected a symptomatically anxious group. State anxiety, or “in-the-moment” anxiety was measured both pre- and post-session. The results showed a reduction in state anxiety after each session, particularly in S1, which could be reflective of the client being able to tell their story and being heard in an affirming way. The scores also revealed that anxiety continued to decrease after each session and also had an overall downward trend across all six sessions. Anxiety is seen as a constant state of bodily stimulation which can be short or long-term (Andreassi, 2007) resulting in a constant appraisal and perception of threat that affects our cognitive and emotional functioning (Charney, 2004). The disordered cognitive and emotional functioning associated with anxiety impacts the functioning of our physiology, especially the endocrine system (Bambling, 2006). Feeling anxious also doubles the risk of coronary heart disease (Todaro et al., 2003). Our study suggests that both thoughts and feelings affect our physiology and that the talking cure of psychotherapy, built on a strong therapeutic alliance, does indeed have a curative effect. This is supported by the above results, which clearly established a reduction in anxiety over the six sessions and appeared to reduce the heart rate or beats per minute (BPM) of clients.
Heart Rate (Hr)
Anxiety and depression increase the rate of heart disease in both men and women (Lett, Blumenthal & Babyak, 2004). Increased HR has also become a crucial indicator of cardiovascular disease (Messerli & Bangalore, 2008). Our results established that HR, which was measured before and after each session, reduced significantly after each therapy session. This decrease in cardiovascular activity was also progressively reflected across all six sessions. The results show that as anxiety reduces so does our heart rate (Guntupalli et al., 2006).
Therapy
Our subjective findings were based on short-term therapy of six weekly sessions, where clients explored a current issue that was related to their anxiety. Short-term therapy can be as effective as long-term therapy for clients to reach an adequate resolution of their issues (Hubble, Duncan & Miller, 1999), where the quality of the therapeutic relationship is a key factor (Houston, 2003).
Six sessions of psychodynamic therapy were found to be as effective as long-term therapy in relation to successful clinical outcome (Messer & Warren, 1995). Using the establishment of a positive therapeutic alliance as a measure, researchers found no outcome differences between 8 and 16 sessions (Stiles et al., 1998). Moreover, brief psychodynamic therapy showed that when therapeutic alliance was clearly established by the third session, and if the therapy had a clear focal approach (Coren, 2001), four sessions were enough for the client to achieve a successful therapeutic outcome (Despland, Drapeau & de Roten, 2005). Consistent with these findings our subjective measurement of therapeutic alliance, the Working Alliance Inventory (WAI), revealed that therapeutic alliance was established by S3.
WAI
The WAI is a validated 36-item self-report measure of TA. The WAI is based on psychodynamic and cognitive behavioural concepts of the alliance (Bordin, 1994). The WAI correlates positively with other TA measures, such as the California Psychotherapy Alliance Scale (Safran & Wallner, 1991); the Helping Alliance and the Vanderbilt Scales (Tichenor & Hill, 1989). The bond subscale of the WAI reliably predicts if clients will continue with therapy after the first session (Marci, Glick & Ablon, 2006).
This exploratory study replicated previous research(Marci et al., 2007) in the development and establishment of TA. We found that TA had developed by S3 and was significantly higher in S3 compared to S6 compared to S1 as the therapy progressed. By session 3 both client and therapist were reporting the same level of ta. So they agreed that therapeutic relationship was in place for both of them. It then continued to climb incrementally over the continuing sessions at the same rate reported by both therapist and client. While the TA increased significantly for the therapist as the sessions developed, Figure 4 shows that therapists perceive TA to be higher in earlier sessions than their clients do.
This reflects that therapists and clients assess TA differently. Therapists estimate it on what they believe should happen, whereas clients appraise the current therapeutic relationship on their experience of past relationships (Horvath & Symonds, 1991). Once clients have established trust through the rapport-building process, the alliance continues to build across the sessions (Heaton, 1998).
Future Directions and Conclusions
The findings of this study have allowed us to identify where the state of therapeutic alliance or deep empathic resonance lies in the brain. By tracking the neural correlates of therapeutic alliance, we have begun to establish the neuroscience of the phenomenon of therapeutic alliance (TA) and its effect on the therapeutic process. This study establishes that certain parts of the brain are linked to the underlying processes of clients as they begin to create a new narrative during moments of high therapeutic alliance. The parietal cortex becomes significantly active while the prefrontal and occipital sites appear to aid this process by becoming less active.
These results have rich implications for clinical intervention. They support the importance of the concept of therapeutic alliance as a clinical tool and its effect on clients’ neurophysiology. It takes the theory of TA’s effect on the therapeutic process and outcome from a subjective discussion to an objective fact.
By understanding the impact of therapy on the neurophysiology of our clients, psychotherapists are better resourced to help clients recognise cognitive, affective patterns that cause maladaptive behaviours based on old neural pathways. This research is beginning to build a common language and theoretical base for better understanding of how and when clients access change and what we therapists can do to foster that change process.
Our study’s major strengths were its triangulated design of a 180-hour repeated-measures design; high participation rate (there were no drop outs); wide age range and multi-cultural population; random selection and triangulated design. Nevertheless although our study contained a large enough sample size to establish critical significant findings, a larger population would have confirmed the trends some of the interesting trends identified as well. Our client sample was restricted to symptomatic anxiety. Further studies could look at larger sample groups presenting more diverse issues, such as affective disorders like depression and other mental health issues.
By cultivating a more informed climate of therapeutic change, through recognising TA as a potent force for change, clinicians now have the opportunity to instigate, recognise and capture these moments of high TA more effectively and to help produce deeper successful client outcome in a more realistic time frame. Experience tells us that time is fast becoming one of the most precious commodities in the 21st century. Neuroscience proves that successful psychotherapeutic outcome involves changes in the brain through the mechanism of neuroplasticity. This research establishes that there are neurophysiological changes in clients during moments of high TA.
Therefore successful psychotherapy is reliant on a positive therapeutic alliance. We conclude that psychotherapy is indeed a talking cure, which impacts the physiological, psychological and neurological levels of client’s awareness.
By tracking a client’s subjective experience through neurophysiology this research provides a common language and a matrix for all therapeutic models to build upon the importance of therapeutic alliance as a clinical construct and its relevance to the change process. It also shows how using neuroscience can impact the therapist’s interpretations of what the client is saying, doing and feeling, therefore, increasing the level of clinical effectiveness. In a society that is increasingly demanding a cost-conscious and service-effective therapy and counselling sector (Street & Downey, 1996), this study adds to the body of neuroscience knowledge that may help to implement more effective therapy.
(2000a). The EEG and behavior: Sensation, attention, perception, conditioning and sleep. In J.L. Andreassi (Ed.), Psychophysiology: Human Behaviour & Psychological Response (pp. 61–84). New Jersey: Lawrence Erlbaum Associates, Inc.Google Scholar
(2000b). Psychophysiology: Human Behaviour & Psychological Response (4th ed.). New Jersey: Lawrence Erlbaum Associates, Inc.Google Scholar
(2007). Psychophysiology: Human Behaviour & Psychological Response (5th ed.), New Jersey: Lawrence Erlbaum Associates, Inc.Google Scholar
(2006). Measures of the therapeutic relationship in severe psychotic illness: a comparison of two scales. International Journal of Social Psychiatry, 52, 256–266.Crossref, Medline, Google Scholar
(2006). Mind, body and heart: Psychotherapy and the relationship between mental and physical health. Psychotherapy in Australia, 12, 52–59.Google Scholar
(2007). Editorial—PACFA NEWS. Psychotherapy & Counselling Federation of Australia, 34, p. 3.Google Scholar
(2006, October). Functional Neuroimaging and pstychoanalytical psychotherapy—can it contribute to our process of change. Paper presented at the Neuro-psychoanalysis lecture series, New York, Psychoanalytical Institute.Google Scholar
1994, Theory and research on the therapeutic working : new directions. In The Working Alliance: Theory, Research and Practicealliance, John Wiley & Sons, New York, NY.Google Scholar
(2002). Role of genotypes in the cycle of violence in maltreated children. Science, 297, 851–857.Crossref, Medline, Google Scholar
(1999). Handbook of attachment: Theory research and clinical applications. New York: Guilford Press.Google Scholar
(2004). Discovering the neural basis of human social anxiety: A diagnostic and therapeutic imperative. American Journal of Psychiatry, 161, 1–2.Crossref, Medline, Google Scholar
(2005). Therapists’ experience of relational depth: A qualitative interview study. Counselling and Psychotherapy Research, 5, 87–95.Crossref, Google Scholar
(2001). Short-term psychotherapy—a psychodynamic approach. New York: Other Press.Google Scholar
(2002). The Neuroscience of Psychotherapy: Building and re-building the human brain. New York:W. W. Norton & Company.Google Scholar
(1996). The lifestyle appraisal questionnaire: a comprehensive assessment of health and stress. Psychology and Health, 11, 331–343.Crossref, Google Scholar
(1997). Neurobiology of fear responses: The role of the amygdala. Journal of Neuropsychiatry and Clinical Neurosciences, 9, 382–402.Crossref, Medline, Google Scholar
(2003). Neural correlates of feeling sympathy. Neuropsychologia, 41, 127–138.Crossref, Medline, Google Scholar
(2005). naturalistic study of the effectiveness of a four session format: The Brief psychodynamic Intervention. Brief Treatment and Crisis Intervention, 5, 368–378.Crossref, Google Scholar
(1964). Incodence of theta rhythm prior to awareness of a visual stimulus, Nature, 203, 167–170.Crossref, Medline, Google Scholar
(2005). Quantifiable change in functional brain response to empathic and forgivability judgements with resolution of posttraumatic stress disorder. Psychiatry Research, 140, 45–53.Crossref, Medline, Google Scholar
(1996). Where in the brain does visual attention select the forest and the trees? Nature, 382, 626–628.Crossref, Medline, Google Scholar
(1991). Spehlmann’s EEG Primer (2nd ed.). Amsterdam: Elsevier Science.Google Scholar
. 1968 - The Dynamics of Transference. In J. Starchey (Ed. & Trans.), The standard edition of the complete psychological works of Sigmund Freud, Vol. 23, pp.174). London: Hogarth Press. (Original work published 1912)Google Scholar
(1989). Marital interaction and satisfaction: A longitudinal view. Journal of Consulting and Clinical Psychology, 57, 47–52.Crossref, Medline, Google Scholar
(2006). Social modulation of pain as evidence for empathy in mice. Science, 312, 1967–1970.Crossref, Medline, Google Scholar
(1967). The technique and practice of psycho-analysis. London: Hogarth Press.Google Scholar
(2006). Psychophysiological responses of adults who do not stutter while listening to stuttering. International Journal of Psychophysiology, 62, 1–8.Crossref, Medline, Google Scholar
(2002). Reliability generalisation of working Alliance Inventory Scale scores. Educational & Psychological Measurement, 62, 659–673.Crossref, Google Scholar
(1998) Establishing rapport: Building basic therapeutic skills, San Francisco: Jossey-Bass.Google Scholar
(1977). Theta bursts: An EEG pattern in normal subjects practising the Transcendental Meditation Technique. Electroencephalography & Clinical Neurophysiology, 42, 397–405.Crossref, Medline, Google Scholar
(2002). Focusing orientated/experiential psychotherapy. In D. CainJ. Seeman (Eds.), Humanistic psychotherapy: Handbook of research and practice, pp. 221–256. Washington, DC: American Psychological Association.Crossref, Google Scholar
(2000). The therapeutic relationship: From transference to alliance. Journal of Clinical Psychology, 56, 163–173.Crossref, Medline, Google Scholar
(1993). The Therapeutic Alliance and its measures. In N.E. MillerL. LuborskyJ.P. BarberJ.P. Docherty (Eds), Psychodynamic treatment research (pp. 247–273). New York: Basic Books.Google Scholar
(1986). The development of the Working Alliance Inventory. In L. GreenbergW. Pinsoff (Eds.), The Psychotherapeutic process: A Resource Handbook (pp. 529–556). New York: Guilford Press.Google Scholar
(1994). The Working Alliance: Theory, Research and Practice. New York:John Wiley & Sons.Google Scholar
(1991). Relation between working alliance and outcome in psychotherapy: A meta-analysis. Journal of Counselling Psychology, 38, 139–149.Crossref, Google Scholar
2003, Brief Gestalt Therapy. London: Sage Publications.Crossref, Google Scholar
(2006). Therapeutic alliance mediates the relationship between interpersonal problems and depression outcome in a cohort of multiple sclerosis patients. Journal of Clinical psychology, 62, 1197–1204.Crossref, Medline, Google Scholar
Hubble, M.A.Duncan, B.L.Miller, S.D. (Eds). (1999). The Heart and Soul of Change: What works in Therapy. American Psychological Association, Washington, DC.Crossref, Google Scholar
(1996). Why you get sick and how you get well: The healing power of feelings, West Hollywood, CA: Dove.Google Scholar
(2000). Effects of therapist self-monitoring on therapeutic alliance and subsequent therapeutic outcome. The Clinical Supervisor, 19, 41Crossref, Google Scholar
(1977). The Restoration of the Self. Madison, CT: International Universities Press.Google Scholar
(1992). Implications of outcome research for psychotherapy integration. In J.C. NorcrossM.R. Goldfried (Eds), Handbook of psychotherapy integration. (pp. 143–189 New York: Basic Books.Google Scholar
(1994). Assessing Psychotherapy outcomes and processes. In A.E. BerginS.L. Garfield (Eds.), Handbook of Psychotherapy and behaviour change (4th edn., pp. 72–113). New York: J. Wiley & Sons.Google Scholar
(2004). Depression as a risk factor for coronary artery disease: evidence, mechanisms and treatment. Psychosomatic Medicine, 66, 305–315.Medline, Google Scholar
(2000). A general theory of love. New York: Random House.Google Scholar
(2008, March). Quest for genius and the search for Einstein’s Brain. Public lecturepresented at the Institute of Neuroscience, University College of London, Newcastle, UK.Google Scholar
(1997). Working with embodiment, energy and resistance. Developing Gestalt Counselling. London: Sage.Google Scholar
(2006). The relationship among patient contemplation, early alliance and continuation in psychotherapy. Psychotherapy Theory, Research, Practice, Training, 43, 238–243.Crossref, Google Scholar
(2007). Physiological correlates of perceived therapist empathy and social-emotional process during psychotherapy. Journal of Nervous and Mental Disease, 195, 103–111.Crossref, Medline, Google Scholar
(1998). Narrative and Psychotherapy. New London, CT: Sage Publications.Google Scholar
(1992). The concept of the therapeutic alliance. Journal of the American Psychoanalytic Association, 40, 1059–1087.Crossref, Medline, Google Scholar
(1995). Models of Brief Psychodynamic Therapy. New York: Guilford Press.Google Scholar
(2008). Resting heart rate and cardiovascular disease: The beta blocker hypertension paradox. Journal of the American College of Cardiology, 51, 330–331.Crossref, Medline, Google Scholar
(1997). Escape from Babel: Toward a unifying language for psychotherapy practice. New York: Norton.Google Scholar
(1999). Discrete time signal processing. Upper Saddle River, NJ: Prentice Hall.Google Scholar
(1998). Affective change processes in therapy for PTSD stemming from childhood abuse. Journal of Psychotherapy Integration, 8, 211–229.Crossref, Google Scholar
(2003). Neuroanatomy of the human affective system’, Brain and Cognition, 52, 24–26.Crossref, Medline, Google Scholar
(1991). The relative predictive validity of two therapeutic alliance measures in cognitive therapy. Psychological assessment. Journal of Consulting and Clinical Psychology, 3, 188–195.Crossref, Google Scholar
(1973). The Treatment Alliance. In the patient and the Analyst. London: Karnac Books.Google Scholar
(1977). EEG theta waves and psychological phenomena: a review and analysis.Biological Psychology, 5, 47–82.Crossref, Medline, Google Scholar
(1994). Affect regulation and the origin of the self: The neurobiology of emotional development. Hillsdale, NJ: Erlbaum.Google Scholar
(2003a). Affect Regulation and the Repair of the Self. New York: W.W. Norton.Google Scholar
(2003b). Revolution Connections. In J. CorrigallH. Wilkinson (Eds.), Psychotherapy and Neuroscience (p. 322). London: Karnac.Google Scholar
(2006). The Mindful Brain. New York: W.W. Norton.Google Scholar
(1990). Cues: The perceptual edge of the transference. International Journal of Psycho-analysis, 71, 219–227.Medline, Google Scholar
(2002). The brain and the inner world—an introduction to the neuroscience of subjective experience. New York: Karnac Books.Google Scholar
(2006). Prevalence and incidence studies of anxiety disorders: A systematic review of the literature. Canadian Journal of Ppsychiatry, 51, 100–113.Crossref, Medline, Google Scholar
(1983). State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.Google Scholar
(2004). The present moment in psychotherapy and everyday life. New York: W.W. Norton.Google Scholar
(1998). lations of the alliance with psychotherapy outcome: Findings in the second Sheffield Psychotherapy Project. Journal of Consulting and Clinical Psychology, 5, 791–802.Crossref, Google Scholar
, (1994). The intersubjective context of intrapsychic experience. In R.D. StolorowG.E. AtwoodB. Brandchaft (Eds.), The intersubjective perspective, Northvale, NJ:Jason Aronson (pp. 3–14.Google Scholar
1987, Psychoanalytic treatment - an intersubjective approach. Hillsdale, N.J.: Analytic Press.Google Scholar
(2009). The neurobiology of the therapeutic relationship between client and therapist: targeting symptomatic anxiety. The Gestalt Journal of Australia and New Zealand, 5, 19–47.Google Scholar
(1996). Brief the rapeutic consultations: An approach to systemic counselling. Chichester, UK: John Wiley & Sons.Google Scholar
(2003). Therapeutic alliance as a measurable psychotherapy skill. Academic Psychiatry, 27, 160–165.Crossref, Medline, Google Scholar
(1989). A comparison of six measures of working alliance. Psychotherapy: Theory Research and Practice, 26, 195–199.Crossref, Google Scholar
(2003). Effect of negative emotions on frequency of coronary heart disease—The normative ageing study. American Journal of Cardiology, 15, 901–906.Google Scholar
(1993). Comparison of QEEG and response accuracy in good vs poorer performers during a vigilant task. International Journal of Psychophysiology, 15, 123–134.Crossref, Medline, Google Scholar
(2008). Centre of attention. Australian Science, 29/1, 28–30.Google Scholar
(1980). Existential Psychotherapy. New York: Basic Books.Google Scholar